- By Jessica Wilson
Maryland lawmakers have introduced legislation aimed at expanding access to comprehensive obesity treatment for low-income residents, a move that comes as debates over the cost and availability of weight-loss medications intensify nationally. Senate Bill 0496, filed on Feb. 2 in Annapolis, would authorize the Maryland Medical Assistance Program to provide full coverage for obesity treatments beginning Jan. 1, 2027, including intensive behavioral therapy, bariatric surgery and any U.S. Food and Drug Administration-approved medication for chronic weight management for people with obesity. The bill also requires the Maryland Department of Health to notify recipients if the state elects to adopt the comprehensive coverage measure, which advocates say could reduce barriers to care for underserved populations disproportionately affected by obesity and related conditions such as diabetes and heart disease.
Obesity remains a major public health challenge in the United States, affecting an estimated 42 percent of adults and contributing to increased risk of chronic illnesses. Research shows that people of color, particularly Black and Hispanic communities, often experience higher obesity prevalence and face structural obstacles to prevention and treatment, including limited access to healthy foods, safe spaces for physical activity and affordable medical care. Expanded coverage under state Medicaid programs could help address these disparities by easing financial burdens and supporting earlier intervention. Health experts emphasize that comprehensive obesity care typically includes lifestyle support, counseling and, when appropriate, medical or surgical options. Providing equitable access to these services is seen as essential to improving long-term health outcomes. Providers and advocates say lack of coverage often forces patients to delay or forgo treatment, with consequences that exacerbate health inequities in marginalized communities.
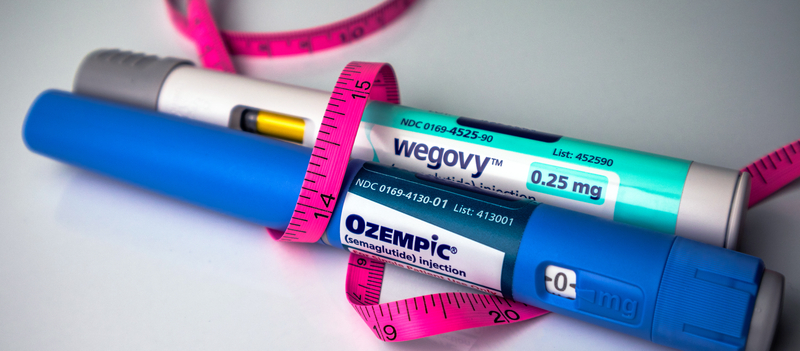
The introduction of SB 0496 coincides with heightened public attention on obesity treatments in the private sector, where pharmaceutical and telehealth companies are grappling with regulatory and legal challenges. In recent days, telehealth provider Hims & Hers Health announced it would withdraw a heavily discounted version of a weight-loss pill that it had priced at $49 for the first month and $99 thereafter, undercutting branded medications such as Wegovy, which typically costs significantly more. The semaglutide-based pill, designed to mimic the active ingredient in Wegovy and similar drugs, drew swift criticism from regulators and manufacturers. Novo Nordisk, maker of Wegovy, threatened legal action and labeled the product unapproved and potentially unsafe, contributing to the decision by Hims & Hers to stop offering the compounded version.
Novo Nordisk’s shares jumped more than 8 percent following the announcement that Hims & Hers was abandoning the low-cost pill, reflecting investor confidence in the company’s efforts to defend its market position and patent rights. The U.S. Food and Drug Administration signaled an intention to tighten oversight of unauthorized compounded glucagon-like peptide-1 (GLP-1) medications, citing concerns about quality, safety and compliance with federal law. Compounded drugs are typically prepared by pharmacists to meet individual patient needs and are not subject to the same approval process as factory-manufactured medications; regulators have repeatedly warned they should not be widely marketed as substitutes for FDA-approved treatments.
The legal dispute over compounded weight-loss pills highlights broader tensions in the obesity treatment landscape, where rising demand has fueled a booming market for GLP-1-based therapies. These drugs, originally developed to treat type 2 diabetes, have gained popularity for weight management after studies showed meaningful average weight loss among trial participants. In December 2025 the FDA approved an oral version of the Wegovy pill, representing a significant development in obesity care and offering a new option beyond injectable formulations. That approval was based on clinical evidence indicating substantial weight reduction in adults with obesity or overweight and at least one related health condition.
Cost remains a key concern, particularly for low-income individuals and communities of color who often face greater barriers to accessing obesity treatments. Experts note that while FDA-approved medications can be effective, their high prices place them out of reach for many patients without comprehensive insurance coverage. This economic divide has fueled interest in lower-cost alternatives and intensified scrutiny of compounding practices that attempt to replicate expensive drugs outside the standard regulatory framework. Public health advocates argue that expanding Medicaid coverage for obesity treatment, as proposed in Maryland’s SB 0496, could help bridge the gap between medical innovation and equitable access, enabling patients to benefit from evidence-based therapies while ensuring safety and quality oversight.
Critics of broader coverage often cite cost concerns and question the long-term effectiveness of pharmaceutical interventions without simultaneous lifestyle and environmental supports. Still, proponents maintain that a comprehensive approach to obesity care, encompassing behavioral support, medication and surgery when appropriate, is essential to address a complex, chronic condition. They argue that legislative actions, such as Maryland’s proposed law, signal a growing recognition among policymakers of the need to treat obesity as a fundamental health priority rather than a cosmetic issue. As the state legislature debates SB 0496 this spring, patient advocacy groups, clinicians and community organizations are engaging in discussions about the policy’s potential to improve outcomes for those most affected by obesity and to reduce health inequities across the population.
In the coming months, the progress of the Maryland bill will be watched closely by public health stakeholders and lawmakers in other states considering similar measures. With obesity continuing to contribute to rising healthcare costs and disparities in chronic disease outcomes, the intersection of state policy, pharmaceutical innovation and community access underscores the challenges and opportunities in expanding equitable treatment options. The unfolding debate illustrates how regulatory, legal and legislative efforts are shaping the future of obesity care in the United States, with direct implications for patient access, safety and long-term health.
Stay Informed. Stay Empowered.
Trending Topics
Features
- Drive Toolkit
Download and distribute powerful vaccination QI resources for your community.
- Health Champions
Sign up now to support health equity and sustainable health outcomes in your community.
- Cancer Early Detection
MCED tests use a simple blood draw to screen for many kinds of cancer at once.
- PR
FYHN is a bridge connecting health information providers to BIPOC communities in a trusted environment.
- Medicare
Discover an honest look at our Medicare system.
- Alliance for Representative Clinical Trials
ARC was launched to create a network of community clinicians to diversify and bring clinical trials to communities of color and other communities that have been underrepresented.
- Reducing Patient Risk
The single most important purpose of our healthcare system is to reduce patient risk for an acute event.
- Jessica Wilson
- Subash Kafle
- Jessica Wilson